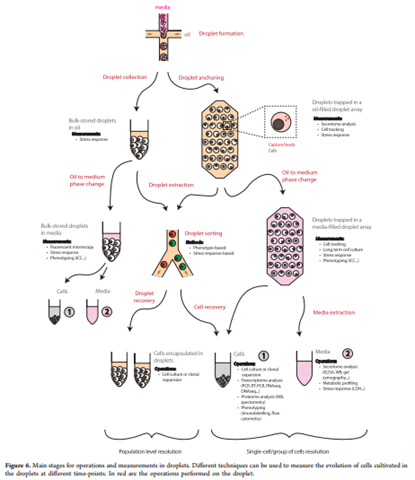
With the ability to easily separate, manipulate, tag, and sequence individual cells, droplet microfluidics technology has contributed to the rapid expansion and maturation of single-cell biological research. At the same time, the very small liquid volumes contained within droplets foster favorable conditions for the study of the cell-to-cell interactions that occur via contact or secreted molecules. High-throughput microfluidics platforms, which allow significantly large numbers of independent replicate experiments to run simultaneously, also help ensure sufficient statistical power for analysis of these cell-to-cell interactions without additional investments of time or resources. In a recent review for the American Chemical Society, Sart et al describe the emerging practice of cell culture within microfluidic droplets, highlight the assays that have been used to characterize cell-to-cell interactions within droplets, and discuss the relevance of cell culture within droplets to applications in cancer biology and immunology.
Quantitative assays that characterize cell viability, stress, proliferation, and gene activity have been adapted for use in microfluidic droplets, making this platform useful for studying cancer cells. While droplets can be collected and analyzed in bulk off-chip, this type of analysis tends to mask the intrapopulation heterogeneity central to a thorough understanding of cancer biology; as a result, assays conducted on droplets (containing either single cells or cultures) are preferred. The main benefit of culturing cancer cells within droplets lies in the ability to model the tumor microenvironment (TME), which has been shown to play a critical role in tumor growth, metastasis, and resistance to cancer therapeutics. Numerous studies have endeavored to create physiologically relevant TME models in droplets by recreating TME composition, three-dimensional architecture, stiffness, rigidity, and biochemical conditions. Tumor models in droplets have also been used to study the process of epithelial-to-mesenchymal transition in two gastric cancers and to screen new anticancer therapeutics, such as the synergistic effect of Paclitaxel, 5-fluorouracil, and flavopiridol. Tumor-associated macrophages, a key component of the TME, have successfully been coencapsulated with human gastric carcinoma cells in droplets and shown to mimic in vivo conditions.
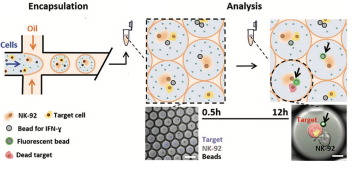
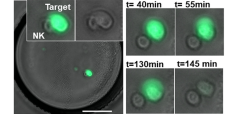
Coencapsulating 3d cancer cell cultures or single cancer cells with immune cells in microfluidic droplets allows the simulation and study of immune-tumor interactions. These interactions have been implicated in playing a central role in tumor progression and response to therapy, and a thorough understanding of these interactions is critical for the development of innovative anti-cancer immunotherapies. Functional screening studies of droplets containing tumor and immune cells using fluorescence microscopy have yielded information about the interplay between interferon-γ secretion and the activity of antitumoral natural killer cells, demonstrated heterogeneity in interaction time, frequency, and killing efficacy of natural killer cells coencapsulated with multiple myeloma and B-cell non-Hodgkin lymphoma cells, and monitored T cell receptor-bearing Jurkat cell activation when in the presence of K652 tumor cells. These studies show the droplet microfluidics platform's potential to not only simulate interactions between immune cells and tumors but to also screen these interactions for functional phenotypes of interest.
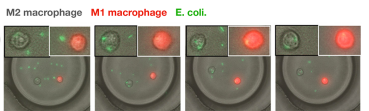
Similarly designed functional screening studies have been used to examine the complex cell-to-cell interactions in a population of mature dendritic cells, demonstrating that not all dendritic cells were able to activate T-cells that were coencapsulated with them, and to explore the interactions between macrophages of distinct phenotypes and E. coli cells. Moreover, 3D models of tissues can be recreated within droplets to better represent in vivo conditions and to explore complex immune cell behaviors such as target searching and cytokine signaling. One study explored the chemical signaling between cultures of Peyer’s patch cells, which participate in intestinal immune surveillance and response, and crypt cells, which compose intestinal glands, demonstrating the importance of Peyer’s patch cell secretions for crypt cell survival. As the practice of cell culture within droplets matures, microfluidics platforms will be poised to help unravel the mysteries of complex interactions between multiple cells. With small droplet volumes that increase the chances for observable interactions to occur and the ability to easily scale experimental design to contain thousands of replicates, droplet microfluidics technology is an essential tool for studying cell-to-cell interactions.



